
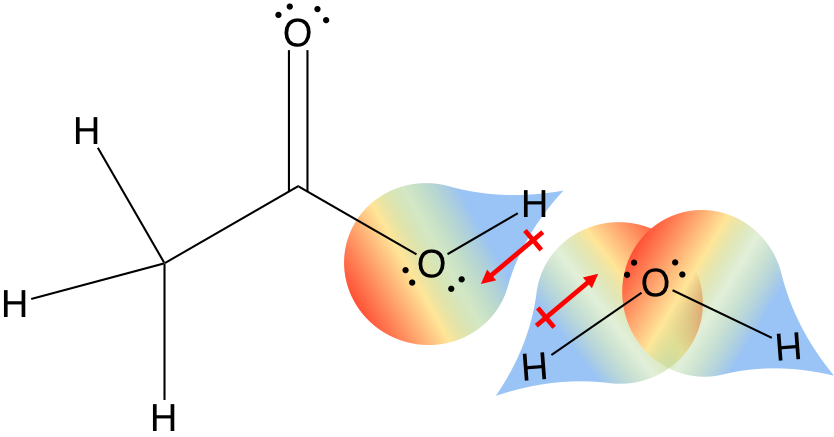
The following diagram shows four possible orientations of the O-H bonds. Since there are two O-H bonds in water, their bond dipoles will interact and may result in a molecular dipole which can be measured. In the case of water, we know that the O-H covalent bond is polar, due to the different electronegativities of hydrogen and oxygen. A molecule which has one or more polar covalent bonds may have a dipole moment as a result of the accumulated bond dipoles. One way in which the shapes of molecules manifest themselves experimentally is through molecular dipole moments. You may examine several Jmol models of compounds discussed above by.
#MOLECULAR DIPOLE MOMENT DEFINITION PC#
A pop-up menu of commands may be accessed by the right button on a PC or a control-click on a Mac while the cursor is inside the display frame. To measure a torsion angle, do a double-click, single-click, single-click, double-click on four atoms. To measure a bond angle, do a double-click, single-click, double-click on three atoms. To measure a distance, double-click on two atoms. Atom distances and angles are easily determined. This powerful visualization tool allows the user to move a molecular stucture in any way desired. Some of the useful features of physical models can be approximated by the model viewing applet Jmol.
#MOLECULAR DIPOLE MOMENT DEFINITION PROFESSIONAL#
Many kinds of model kits are available to students and professional chemists. The best way to study the three-dimensional shapes of molecules is by using molecular models. Click on the university name to visit their site. Nice treatments of VSEPR theory have been provided by Oxford and Purdue. The compound boron trifluoride, BF 3, does not have non-bonding valence electrons and the configuration of its atoms is trigonal. Of course, it is the configuration of atoms (not electrons) that defines the the shape of a molecule, and in this sense ammonia is said to be pyramidal (not tetrahedral). The measured bond angles of these compounds (H 2O 104.5º & NH 3 107.3º) show that they are closer to being tetrahedral than trigonal or linear. In each case there are four regions of electron density associated with the valence shell so that a tetrahedral bond angle is expected. For molecules of water and ammonia, however, the non-bonding electrons must be included in the calculation. In the three examples shown above, the central atom (carbon) does not have any non-bonding valence electrons consequently the configuration may be estimated from the number of bonding partners alone. The bonding configurations of carbon are easy to remember, since there are only three categories. This simple model is based on the fact that electrons repel each other, and that it is reasonable to expect that the bonds and non-bonding valence electron pairs associated with a given atom will prefer to be as far apart as possible. īonding configurations are readily predicted by valence-shell electron-pair repulsion theory, commonly referred to as VSEPR in most introductory chemistry texts. The following examples make use of this notation, and also illustrate the importance of including non-bonding valence shell electron pairs (colored blue) when viewing such configurations. a covalent bond that is partially formed or partially broken). Some texts and other sources may use a dashed bond in the same manner as we have defined the hatched bond, but this can be confusing because the dashed bond is often used to represent a partial bond (i.e. A wedge shaped bond is directed in front of this plane (thick end toward the viewer), as shown by the bond to substituent B and a hatched bond is directed in back of the plane (away from the viewer), as shown by the bond to substituent D. The two bonds to substituents A in the structure on the left are of this kind. As defined in the diagram on the right, a simple straight line represents a bond lying approximately in the surface plane. In most cases the focus of configuration is a carbon atom so the lines specifying bond directions will originate there. In order to represent such configurations on a two-dimensional surface (paper, blackboard or screen), we often use perspective drawings in which the direction of a bond is specified by the line connecting the bonded atoms. This shape is dependent on the preferred spatial orientation of covalent bonds to atoms having two or more bonding partners.Three dimensional configurations are best viewed with the aid of models. The three dimensional shape or configuration of a molecule is an important characteristic.


 0 kommentar(er)
0 kommentar(er)
